

| Product Name | NebuPeptide™ Pegcetacoplan (Peptide Reference) |
|---|---|
| Catalog Number | NBS-233776 |
| Alias/Synonyms | |
| Appearance | Solid |
| Molecule Weight | 43520.1 |
| CAS | 2019171-69-6 |
| Solubility | see COA |
| Storage | Store at -20℃ |
| Shelf Life | see COA |
| Additional info 1 | |
| Additional info 2 |
Product Name:
NebuPeptide™ Pegcetacoplan (Peptide Reference)
Catalog Number:
NBS-233776
Description:
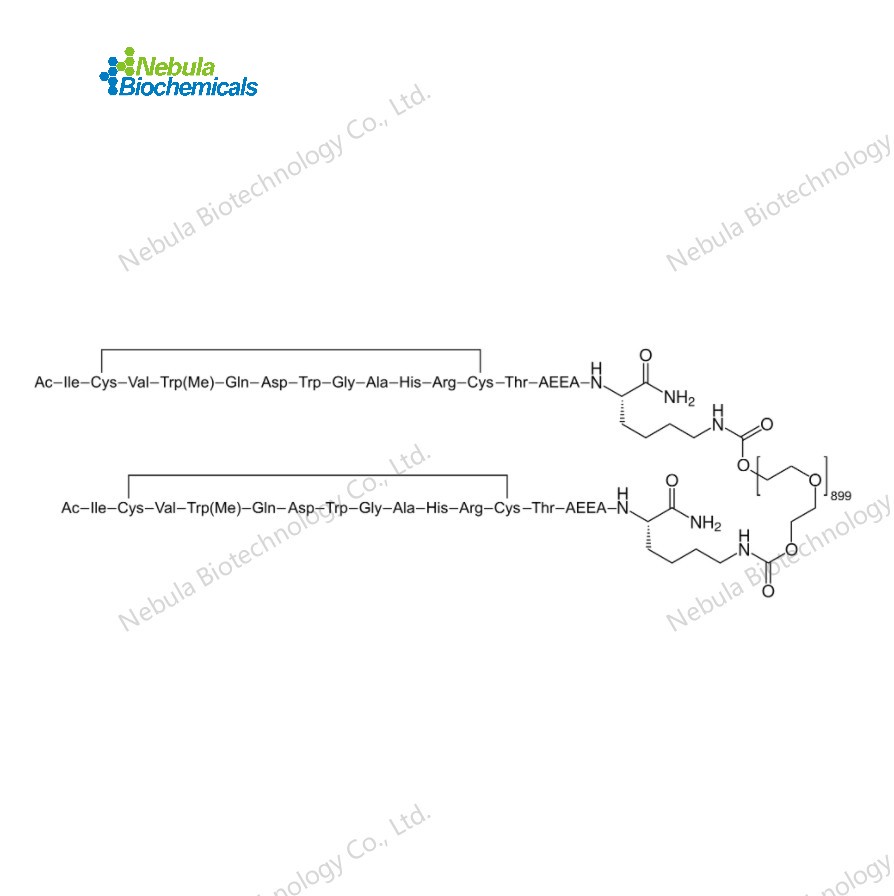
NebuPeptide™ Pegcetacoplan (Peptide Reference)(CAT#NBS-233776) is synthesized in accordance with the Pegcetacoplan sequence and can be utilized for a variety of in vivo and in vitro studies, including quality control, bioactivity verification, immunization, drug development, structural analysis, etc. Nebula Biotechnology Co., Ltd. is capable of providing Lutetium (177Lu) oxodotreotide reference peptide ranging from milligrams to kilograms.
Peptide Name:
Pegcetacoplan
Background:
Pegcetacoplan, sold under the brand name Empaveli, among others, is a medication used to treat paroxysmal nocturnal hemoglobinuria and geographic atrophy of the retina. Pegcetacoplan is a complement inhibitor. Paroxysmal nocturnal hemoglobinuria is characterized by red blood cell destruction, anemia (red blood cells unable to carry enough oxygen to tissues), blood clots, and impaired bone marrow function (not making enough blood cells). Pegcetacoplan is the first treatment for paroxysmal nocturnal hemoglobinuria that binds to and inhibits complement protein C3. Pegcetacoplan was approved for medical use in the United States in May 2021. The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.
Alias:
N/A
CAS Number:
2019171-69-6
Formula:
C1970H3848N50O947S4
Molecular Weight:
43520.1
Purity:
see COA
Solubility:
see COA
Storage & Shipment:
Store at -20℃; ship with blue ice.
For R&D use only!
To get more information, please contact us freely.