

In today's rapidly evolving biomedical research landscape, time-to-market and experimental reliability are paramount. Whether you're engaged in drug discovery, therapeutic antibody development, cell therapy innovation, or large-scale biopharmaceutical production, the quality of your cellular tools can make or break your project's success. Stable cell lines—genetically engineered to consistently express target proteins over long-term culture—have become indispensable workhorses across the R&D continuum. Unlike transient expression systems, a well-developed stable cell line provides a uniform, reproducible, and scalable platform essential for high-throughput screening, functional assays, toxicity testing, and GMP-compliant recombinant protein manufacturing.
However, the journey from gene of interest to a fully characterized, high-expressing, and phenotypically stable monoclonal cell line is fraught with technical challenges. It demands specialized expertise, significant time investment, and rigorous optimization across multiple variables—from vector design and transfection methods to clonal selection and stability validation. Many research teams and biotech startups find this process draining internal resources, delaying critical milestones, and introducing unpredictable bottlenecks.
This is where a specialized stable cell line development service delivers transformative value. By leveraging proven platforms, deep cell line expertise, and industrialized workflows, professional service providers can dramatically compress development timelines, enhance expression yields, and guarantee regulatory-ready documentation—freeing your team to focus on core scientific innovation and accelerating your overall drug development trajectory.
▌The Critical Path: A Detailed Look at Professional Stable Cell Line Development
Constructing a robust stable cell line is a multi-stage scientific endeavor where each phase builds upon the last. Here, we break down the core workflow and the technical precision applied at every step.
1. Strategic Vector Design & Molecular Engineering
The foundation of a successful cell line lies in its genetic blueprint. Off-the-shelf vectors often yield suboptimal results. Professional services begin with a consultative approach to design or select the optimal expression construct for your specific protein and application.
● Promoter & Enhancer Selection: The choice of promoter (e.g., CMV, EF1α, CAG) is tailored to the host cell type (CHO, HEK293, etc.) and desired expression level. Enhancer elements may be incorporated to boost transcriptional activity.
● Selection System Optimization: The appropriate resistance marker (puromycin, neomycin/G418, hygromycin, blasticidin) is chosen based on host cell sensitivity and selection stringency requirements.
● Expression Optimization Elements: Key sequences like the Kozak consensus (for translation initiation), WPRE (for mRNA stabilization), and synthetic polyA signals are integrated to maximize mRNA stability and translational efficiency.
● Advanced Configurations: For toxic proteins or precise control, inducible systems (e.g., Tet-On/Off), bicistronic vectors, or strategies for multi-gene co-expression are engineered. This stage ensures the genetic payload is designed for success before it ever enters a cell.
2. Precision Delivery & Genetic Integration
Efficiently introducing the vector into the host genome is critical. Services employ and optimize a range of delivery methods, selecting the most effective for your cell line.
● Chemical Transfection: Advanced lipid-based reagents are used for their efficiency and ease of use, particularly in amenable cell lines.
● Electroporation: This physical method is often preferred for hard-to-transfect cells, such as primary cells or certain suspension lines, as it facilitates high-efficiency DNA entry.
● Viral Transduction: For exceptionally low transfection efficiency cell types, lentiviral or retroviral vectors offer near-100% delivery rates, ensuring a large pool of transduced cells for downstream screening.
The goal is to generate a vast, diverse population of stably transfected cells, providing the raw material for identifying rare, high-performing clones.
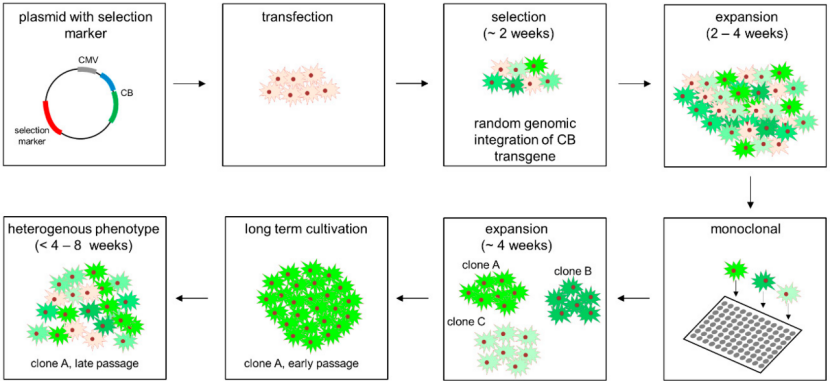
3. Systematic Selection & Monoclonal Isolation
Post-transfection, cells undergo selective pressure to eliminate non-integrated populations. This is followed by the most critical step for long-term consistency: ensuring monoclonality.
● Antibiotic Selection: Cells are cultured in medium containing the appropriate antibiotic, typically for 1-2 weeks, to create a polyclonal pool of resistant cells.
● Guaranteed Monoclonality: To comply with FDA and EMA guidelines for biologics production, evidence of single-cell origin is mandatory. Services use:
● Limiting Dilution: The gold-standard method, where cells are serially diluted to statistically ensure single cells per well, accompanied by meticulous microscopic documentation.
● Fluorescence-Activated Cell Sorting (FACS): For faster isolation, cells can be sorted directly into plates based on a reporter signal (e.g., GFP), with instrument logs providing proof of monoclonality.
This step is non-negotiable for creating a genetically uniform foundation.
4. High-Throughput Screening & Multi-Tiered Validation
Identifying the optimal clone requires sifting through hundreds of monoclonal candidates. A tiered screening strategy balances throughput with depth of analysis.
● Tier 1: Rapid Titer Screening (Days 7-14): Using 96- or 384-well plate formats, supernatant from hundreds of clones is quickly assessed via ELISA or automated flow cytometry to quantify protein expression. The top 5-10% are advanced.
● Tier 2: Small-Scale Expansion & Stability Assessment (Weeks 3-5): Selected clones are expanded in multi-well plates or shake flasks. Productivity (e.g., pg/cell/day) is measured, and an early stability test—passaging cells for several generations without selection pressure—begins to identify clones with durable expression.
● Tier 3: Bioprocess Characterization & Functional Validation (Weeks 6-8): Lead clones undergo deeper analysis:
● Growth & Metabolic Profiling: Assessment of viability, doubling time, and metabolic rates in relevant culture conditions.
● Genetic Stability: PCR or Southern blot analysis to confirm vector integration site and copy number.
● Product Quality: Basic analytics (SDS-PAGE, western blot, activity assays) confirm protein integrity, correct folding, and post-translational modifications (e.g., glycosylation).
Application-Specific Functional Assays: The protein is tested in its intended final assay (e.g., receptor binding, enzyme kinetics, in vitro potency).
5. Cell Banking & Comprehensive Quality Control
The final, validated clone is preserved for future use under the highest standards.
● Cell Bank Creation: A two-tiered banking system is established. A Master Cell Bank (MCB) is produced from the original clone, and from it, multiple Working Cell Banks (WCBs) are derived for routine R&D or production use.
● Rigorous QC Testing: Each bank undergoes a battery of tests, which may include:
● Identity: Short Tandem Repeat (STR) profiling to uniquely identify the cell line and detect cross-contamination.
● Sterility: Tests for bacteria, fungi, and mycoplasma.
● Viral Safety: In vitro and in vivo assays for adventitious viruses.
● Viability & Recovery: Post-thaw performance is verified.
This package ensures the cell line is fully characterized, secure, and ready for scalable expansion or regulatory filings.
▌The Strategic Advantage: Why Outsourcing Accelerates Innovation
Building capability in-house requires capital investment, hiring specialized staff, and enduring a steep learning curve. Partnering with an expert service provides immediate, tangible benefits:
● Radically Shortened Timelines: What typically takes an internal team 6-12 months of iterative optimization can be condensed to 3-4 months through pre-optimized platforms and parallel processing of clones.
● Higher Success Rates & Expression Titers: Experience with diverse proteins and cell lines allows providers to anticipate pitfalls and apply targeted optimizations, consistently achieving higher specific productivity and volumetric yields.
● Regulatory Compliance & Documentation: Services provide complete, audit-ready documentation—from monoclonality evidence to full QC reports—which is invaluable for IND/IMPD/BLA submissions.
● Access to Specialized Technology: Gain the benefits of automated cell pickers, high-content screeners, and advanced analytics without the capital expenditure.
Resource Liberation: Your scientists can dedicate their efforts to upstream target biology and downstream process development, rather than the repetitive, labor-intensive tasks of clone screening.
▌Driving Breakthroughs Across the Biotech Ecosystem
The applications of professionally developed stable cell lines are vast and critical to modern biotechnology:
● Biotherapeutics Manufacturing: CHO cell lines remain the industry standard for producing monoclonal antibodies, fusion proteins, and enzymes. A high-producing, stable clone is the cornerstone of any commercial process.
● Drug Discovery Platforms: Engineered cell lines expressing disease targets (GPCRs, ion channels, kinases) coupled with luciferase or fluorescent reporters are the engine of high-throughput screening campaigns, identifying promising lead compounds.
● Viral Vector Production for Cell & Gene Therapy: The safe and efficient production of lentivirus (LV) or adeno-associated virus (AAV) for CAR-T or gene therapy relies entirely on stable packaging and producer cell lines.
● Disease Modeling & Toxicology: Isogenic cell lines with precise genetic modifications (knock-in, knock-out) enable the study of disease mechanisms in a controlled setting. Reporter cell lines for specific toxicity pathways (e.g., genotoxicity, stress response) are vital for early safety screening.
▌Conclusion: Building on a Foundation of Excellence
In the competitive race to bring new therapies to market, efficiency and reliability in the earliest stages of R&D create a decisive advantage. Stable cell line development is not merely a service but a strategic partnership that de-risks your pipeline and accelerates your critical path.
By entrusting this complex, foundational work to specialists, you secure more than just a vial of cells. You gain a validated, high-performance biological tool, comprehensive data for decision-making, and the most precious resource of all: time. This enables you to move faster from concept to candidate, from discovery to development, and ultimately, to deliver transformative medicines to patients in need.