
 小红书 每周二三四19:30-20:30直播
小红书 每周二三四19:30-20:30直播

 小红书 每周二三四19:30-20:30直播
小红书 每周二三四19:30-20:30直播
辰辉创聚生物®Nebulabio 深耕 ADC 小分子领域多年,主要提供 ADC 药物小分子以及 ADC 偶联定制服务,拥有 80多种库存现货产品。
ADC药物是采用特定的连接子将抗体和小分子细胞毒药物连接起来,其主要组成成分包括 抗体、连接子和小分子细胞毒药物(smallmolecular cytotoxic drug,SM)。抗体分子主要发挥靶向投递 作用,小分子药物发挥效应。但有些抗体同时存在 抗肿瘤的药效学作用,如Kadcyla中阿多曲妥珠单抗(ado-trastuzumab)和美坦新(maitansine)(美登素)存在协同作用。
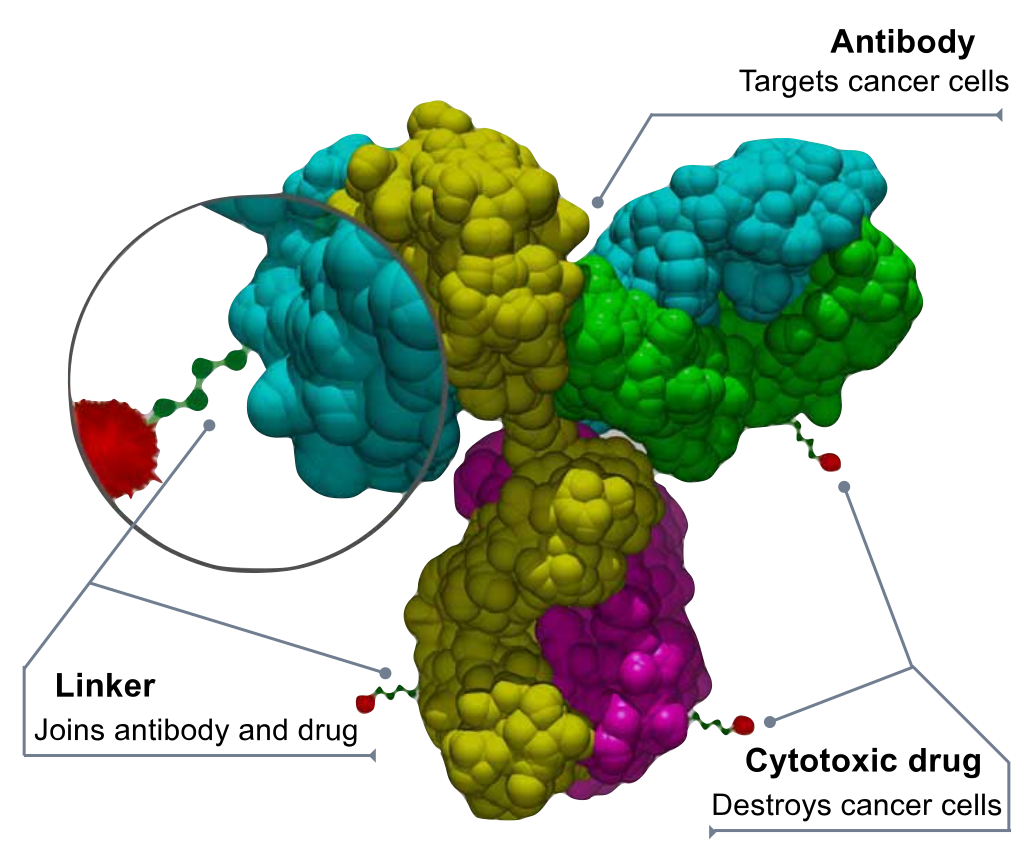
系列产品列表如下:
| 目录号 | 品名 | 物种 | 靶点 | 表达细胞系 |
| NBR-1000 | NebuSelect™ Anti-ALCAM / CD166 Reference Antibody (Praluzatamab-MMAE) | Human | ALCAM / CD166 | CHO |
| NBR-1001 | NebuSelect™ Anti-AMHR2 Reference Antibody (Murlentamab-MMAE) | Human | AMHR2 | CHO |
| NBR-1002 | NebuSelect™ Anti-AXL / UFO Reference Antibody (Enapotamab vedotin) | Human | AXL / UFO | CHO |
| NBR-1003 | NebuSelect™ Anti-AXL / UFO Reference Antibody (Tilvestamab-MMAE) | Human | AXL / UFO | CHO |
| NBR-1004 | NebuSelect™ Anti-MUC16 Reference Antibody (oregovomab-MMAE) | Human | CA125 / MUC16 | CHO |
| NBR-1005 | NebuSelect™ Anti-MUC16 Reference Antibody (Sofituzumab vedotin) | Human | CA125 / MUC16 | CHO |
| NBR-1006 | NebuSelect™ Anti-CA9 / CAIX Reference Antibody (girentuximab-MMAE) | Human | CA9 / Carbonic anhydrase 9 | CHO |
| NBR-1007 | NebuSelect™ Anti-CD19 Reference Antibody (denintuzumab-MMAF) | Human | CD19 | CHO |
| NBR-1008 | NebuSelect™ Anti-CD79b Reference Antibody (Polatuzumab vedotin-piiq) | Human | CD79b | CHO |
| NBR-1009 | NebuSelect™ Anti-CD79b Reference Antibody (iladatuzumab vedotin) | Human | CD79b | CHO |
| NBR-1010 | NebuSelect™ Anti-CEACAM5 / CEA / CD66e Reference Antibody (labetuzumab govitecan) | Human | CEACAM5 / CEA / CD66e | CHO |
| NBR-1011 | NebuSelect™ Anti-CEACAM5 / CEA / CD66e Reference Antibody (Tusamitamab-MMAE) | Human | CEACAM5 / CEA / CD66e | CHO |
| NBR-1012 | NebuSelect™ Anti-CLDN18.2 Reference Antibody (zolbetuximab MMAE) | Human | CLDN18.2 | CHO |
| NBR-1013 | NebuSelect™ Anti-CXCR4/CD184 Reference Antibody (ulocuplumab-MMAE) | Human | CXCR4 / CD184 | CHO |
| NBR-1014 | NebuSelect™ Anti-DLL3 Reference Antibody (rovalpituzumab-MMAE) | Human | DLL3 | CHO |
| NBR-1015 | NebuSelect™ Anti-Endoglin / CD105 Reference Antibody (carotuximab-MMAE) | Human | Endoglin / CD105 | CHO |
| NBR-1016 | NebuSelect™ Anti-ERBB1 / EGFR / HER1 Reference Antibody (depatuxizumab-MMAF) | Human | ERBB1 / EGFR / HER1 | CHO |
| NBR-1017 | NebuSelect™ Anti-ERBB1 / EGFR / HER1 Reference Antibody (Cetuximab-MMAE) | Human | ERBB1 / EGFR / HER1 | CHO |
| NBR-1018 | NebuSelect™ Anti-ERBB2 / HER2 / CD340 Reference Antibody (Disitamab vedotin) | Human | ERBB2 / HER2 / CD340 | CHO |
| NBR-1019 | NebuSelect™ Anti-ERBB2 / HER2 / CD340 Reference Antibody (Trastuzumab-MMAE) | Human | ERBB2 / HER2 / CD340 | CHO |
| NBR-1020 | NebuSelect™ Anti-ERBB3/ HER3 Reference Antibody (patritumab-MMAE) | Human | ERBB3 / HER3 | CHO |
| NBR-1021 | NebuSelect™ Anti-ERBB3/ HER3 Reference Antibody (Patritumab deruxtecan) | Human | ERBB3 / HER3 | CHO |
| NBR-1022 | NebuSelect™ Anti-FOLH1 / PSMA Reference Antibody (rosopatamab-MMAE) | Human | FOLH1 / PSMA | CHO |
| NBR-1023 | NebuSelect™ Anti-FOLR1 / FRA Reference Antibody (farletuzumab-MMAE) | Human | FOLR1 / FRA | CHO |
| NBR-1024 | NebuSelect™ Anti-FOLR1 / FRA Reference Antibody (mirvetuximab-MMAE) | Human | FOLR1 / FRA | CHO |
| NBR-1025 | NebuSelect™ Anti-GD3 Reference Antibody (ecromeximab-MMAE) | Human | GD3 | CHO |
| NBR-1026 | NebuSelect™ Anti-GPC3 / Glypican-3 Reference Antibody (Codrituzumab-MMAE) | Human | GPC3 / Glypican-3 | CHO |
| NBR-1027 | NebuSelect™ Anti-GPNMB Reference Antibody (Glembatumumab vedotin) | Human | GPNMB | CHO |
| NBR-1028 | NebuSelect™ Anti-GUCY2C Reference Antibody (Indusatumab vedotin) | Human | GUCY2C | CHO |
| NBR-1029 | NebuSelect™ Anti-HGFR / c-Met Reference Antibody (Telisotuzumab vedotin) | Human | HGFR / c-Met | CHO |
| NBR-1030 | NebuSelect™ Anti-IL-3Ra / CD123 Reference Antibody (talacotuzumab-MMAE) | Human | IL-3Ra / CD123 | CHO |
| NBR-1031 | NebuSelect™ Anti-KAAG1 Reference Antibody (ADCT-901-MMAE) | Human | KAAG1 | CHO |
| NBR-1032 | NebuSelect™ Anti-LIV-1 / SLC39A6 Reference Antibody (Ladiratuzumab vedotin) | Human | LIV-1 / SLC39A6 | CHO |
| NBR-1033 | NebuSelect™ Anti-Mesothelin Reference Antibody (anetumab-MMAE) | Human | Mesothelin | CHO |
| NBR-1034 | NebuSelect™ Anti-MU5AC Reference Antibody (ensituximab-MMAE) | Human | MU5AC | CHO |
| NBR-1035 | NebuSelect™ Anti-MUC1 Reference Antibody (clivatuzumab-MMAE) | Human | MUC1 | CHO |
| NBR-1036 | NebuSelect™ Anti-NaPi2b / SLC34A2 Reference Antibody (Lifastuzumab vedotin) | Human | NaPi2b / SLC34A2 | CHO |
| NBR-1037 | NebuSelect™ Anti-NCAM1 / CD56 Reference Antibody (lorvotuzumab-MMAE) | Human | NCAM1 / CD56 | CHO |
| NBR-1038 | NebuSelect™ Anti-Nectin-4 Reference Antibody (enfortumab vedotin-ejfv) | Human | Nectin-4 | CHO |
| NBR-1039 | NebuSelect™ Anti-ROR1 Reference Antibody (Zilovertamab vedotin) | Human | ROR1 | CHO |
| NBR-1040 | NebuSelect™ Anti-ROR2 Reference Antibody (ozuriftamab vedotin) | Human | ROR2 | CHO |
| NBR-1041 | NebuSelect™ Anti-Siglec-2 / CD22 Reference Antibody (Pinatuzumab vedotin) | Human | Siglec-2 / CD22 | CHO |
| NBR-1042 | NebuSelect™ Anti-Siglec-2 / CD22 Reference Antibody (inotuzumab-MMAE) | Human | Siglec-2 / CD22 | CHO |
| NBR-1043 | NebuSelect™ Anti-SLITRK6 Reference Antibody (Sirtratumab vedotin) | Human | SLITRK6 | CHO |
| NBR-1044 | NebuSelect™ Anti-TF / Factor III / Tissue Factor / CD142 Reference Antibody (tisotumab vedotin) | Human | TF / Factor III / Tissue Factor / CD142 | CHO |
| NBR-1045 | NebuSelect™ Anti-TNFRSF8 / CD30 Reference Antibody (brentuximab vedotin) | Human | TNFRSF8 / CD30 | CHO |
| NBR-1046 | NebuSelect™ Anti-TNFSF7 / CD27L / CD70 Reference Antibody (Vorsetuzumab mafodotin) | Human | TNFSF7 / CD27L / CD70 | CHO |
| NBR-1047 | NebuSelect™ Anti-TROP2 Reference Antibody (datopotamab deruxtecan) | Human | TROP2 | CHO |
| NBR-1048 | NebuSelect™ Anti-TROP2 Reference Antibody (Sacituzumab-MMAE) | Human | TROP2 | CHO |
关键词
Praluzatamab Biosimilar; Murlentamab Biosimilar; Enapotamab Biosimilar; Tilvestamab Biosimilar; oregovomab Biosimilar; Sofituzumab Biosimilar; girentuximab Biosimilar; denintuzumab Biosimilar; Polatuzumab Biosimilar; iladatuzumab Biosimilar; labetuzumab Biosimilar; Tusamitamab Biosimilar; zolbetuximab Biosimilar; ulocuplumab Biosimilar; rovalpituzumab Biosimilar; carotuximab Biosimilar; depatuxizumab Biosimilar; Cetuximab Biosimilar; Disitamab Biosimilar; Trastuzumab Biosimilar; patritumab Biosimilar; Patritumab Biosimilar; rosopatamab Biosimilar; farletuzumab Biosimilar; mirvetuximab Biosimilar; ecromeximab Biosimilar; Codrituzumab Biosimilar; Glembatumumab Biosimilar; Indusatumab Biosimilar; Telisotuzumab Biosimilar; talacotuzumab Biosimilar; ADCT-901 Biosimilar; Ladiratuzumab Biosimilar; anetumab Biosimilar; ensituximab Biosimilar; clivatuzumab Biosimilar; Lifastuzumab Biosimilar; lorvotuzumab Biosimilar; enfortumab Biosimilar; Zilovertamab Biosimilar; ozuriftamab Biosimilar; Pinatuzumab Biosimilar; inotuzumab Biosimilar; Sirtratumab Biosimilar; tisotumab Biosimilar; brentuximab Biosimilar; Vorsetuzumab Biosimilar; datopotamab Biosimilar; Sacituzumab Biosimilar; Praluzatamab 生物类似药; Murlentamab 生物类似药; Enapotamab 生物类似药; Tilvestamab 生物类似药; oregovomab 生物类似药; Sofituzumab 生物类似药; girentuximab 生物类似药; denintuzumab 生物类似药; Polatuzumab 生物类似药; iladatuzumab 生物类似药; labetuzumab 生物类似药; Tusamitamab 生物类似药; zolbetuximab 生物类似药; ulocuplumab 生物类似药; rovalpituzumab 生物类似药; carotuximab 生物类似药; depatuxizumab 生物类似药; Cetuximab 生物类似药; Disitamab 生物类似药; Trastuzumab 生物类似药; patritumab 生物类似药; Patritumab 生物类似药; rosopatamab 生物类似药; farletuzumab 生物类似药; mirvetuximab 生物类似药; ecromeximab 生物类似药; Codrituzumab 生物类似药; Glembatumumab 生物类似药; Indusatumab 生物类似药; Telisotuzumab 生物类似药; talacotuzumab 生物类似药; ADCT-901 生物类似药; Ladiratuzumab 生物类似药; anetumab 生物类似药; ensituximab 生物类似药; clivatuzumab 生物类似药; Lifastuzumab 生物类似药; lorvotuzumab 生物类似药; enfortumab 生物类似药; Zilovertamab 生物类似药; ozuriftamab 生物类似药; Pinatuzumab 生物类似药; inotuzumab 生物类似药; Sirtratumab 生物类似药; tisotumab 生物类似药; brentuximab 生物类似药; Vorsetuzumab 生物类似药; datopotamab 生物类似药; Sacituzumab 生物类似药; 科研级 Praluzatamab; 科研级 Murlentamab; 科研级 Enapotamab; 科研级 Tilvestamab; 科研级 oregovomab; 科研级 Sofituzumab; 科研级 girentuximab; 科研级 denintuzumab; 科研级 Polatuzumab; 科研级 iladatuzumab; 科研级 labetuzumab; 科研级 Tusamitamab; 科研级 zolbetuximab; 科研级 ulocuplumab; 科研级 rovalpituzumab; 科研级 carotuximab; 科研级 depatuxizumab; 科研级 Cetuximab; 科研级 Disitamab; 科研级 Trastuzumab; 科研级 patritumab; 科研级 Patritumab; 科研级 rosopatamab; 科研级 farletuzumab; 科研级 mirvetuximab; 科研级 ecromeximab; 科研级 Codrituzumab; 科研级 Glembatumumab; 科研级 Indusatumab; 科研级 Telisotuzumab; 科研级 talacotuzumab; 科研级 ADCT-901; 科研级 Ladiratuzumab; 科研级 anetumab; 科研级 ensituximab; 科研级 clivatuzumab; 科研级 Lifastuzumab; 科研级 lorvotuzumab; 科研级 enfortumab; 科研级 Zilovertamab; 科研级 ozuriftamab; 科研级 Pinatuzumab; 科研级 inotuzumab; 科研级 Sirtratumab; 科研级 tisotumab; 科研级 brentuximab; 科研级 Vorsetuzumab; 科研级 datopotamab; 科研级 Sacituzumab; Praluzatamab; Murlentamab; Enapotamab; Tilvestamab; oregovomab; Sofituzumab; girentuximab; denintuzumab; Polatuzumab; iladatuzumab; labetuzumab; Tusamitamab; zolbetuximab; ulocuplumab; rovalpituzumab; carotuximab; depatuxizumab; Cetuximab; Disitamab; Trastuzumab; patritumab; Patritumab; rosopatamab; farletuzumab; mirvetuximab; ecromeximab; Codrituzumab; Glembatumumab; Indusatumab; Telisotuzumab; talacotuzumab; ADCT-901; Ladiratuzumab; anetumab; ensituximab; clivatuzumab; Lifastuzumab; lorvotuzumab; enfortumab; Zilovertamab; ozuriftamab; Pinatuzumab; inotuzumab; Sirtratumab; tisotumab; brentuximab; Vorsetuzumab; datopotamab; Sacituzum