

In today’s rapidly evolving biopharmaceutical landscape, stable cell lines are a foundational enabling technology for biologics research, development, and manufacturing. They provide consistent, long-term expression of target proteins—including monoclonal antibodies, recombinant enzymes, viral vectors, and complex membrane proteins—supporting scalable production, reproducible screening, and rigorous functional studies.
The path from a gene of interest to a high-producing, genetically stable monoclonal cell line is technically complex and resource intensive. Nebulabio’s stable cell line development services are designed to remove this complexity. By combining advanced gene integration technologies, high-throughput clone screening, and deep cell engineering expertise, we deliver a predictable, efficient, and accelerated development pathway from discovery to application.
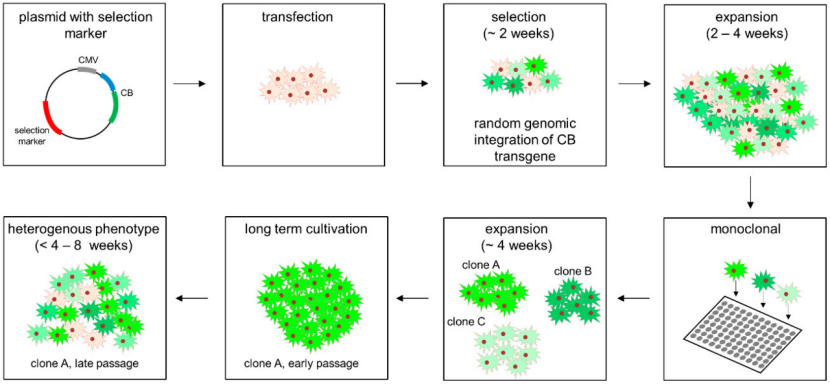
▌ The Critical Role of Stable Cell Lines
Stable cell lines generated through genomic integration of a transgene offer clear advantages over transient expression systems:
✎ Reliable, long-term protein production for research, preclinical development, and commercial manufacturing
✎ High product consistency and homogeneity derived from clonally validated cell populations
✎ A robust foundation for upstream process development and scale-up to bioreactor production
✎ Improved cost efficiency by eliminating repeated transient transfections for long-term programs
▌ A Systematic Framework for Stable Cell Line Development
✎ Vector and Host Cell Strategy
Nebulabio designs optimized expression constructs incorporating high-performance promoters, secretion signal peptides, appropriate selection systems, and regulatory elements to enhance transcriptional stability and expression durability.
Host cell selection is guided by product requirements and downstream application:
•HEK293 systems for rapid expression of research proteins, antigens, and viral vectors
•CHO platforms, including CHO-K1 and CHO-S, as the industry standard for therapeutic protein production with human-like glycosylation and proven scalability in serum-free suspension culture
•Specialized cell lines, such as Vero cells for vaccine production or insect cells for select recombinant proteins
✎ Precision Gene Delivery and Genomic Integration
Stable and reproducible expression is achieved using optimized gene delivery strategies:
•Lentiviral transduction for high-efficiency integration in dividing and non-dividing cells, enabling rapid generation of stable expression pools
•Site-specific integration technologies, including CRISPR/Cas9 and Flp-In systems, targeting genomic safe-harbor loci to minimize positional effects and gene silencing
•Advanced non-viral transfection methods for applications requiring plasmid-based integration
✎ Clone Selection and Monoclonality Assurance
Following selection, high-performing clones are identified through a rigorous screening cascade:
•Single-cell isolation by limiting dilution or fluorescence-activated cell sorting, supported by image-based documentation to ensure regulatory-grade proof of monoclonality
•High-throughput clone screening using automated ELISA, HTRF, and flow-based assays to rapidly identify top-expressing clones
✎ Stability Evaluation and Product Characterization
Lead clones undergo extensive evaluation to confirm long-term performance:
•Growth and expression stability studies across extended passages in the absence of selection pressure
•Comprehensive product characterization including SDS-PAGE, western blotting, peptide mapping, glycosylation profiling, and functional bioactivity assays
✎ Cell Banking and Quality Control
The final selected clone is expanded to generate fully characterized master and working cell banks. Quality control testing includes cell line authentication, sterility and mycoplasma testing, and adventitious agent screening, ensuring suitability for regulated manufacturing or critical research applications.
▌ Nebulabio Signature Technology Platforms
✎ GFP/FACS-Based High-Efficiency Enrichment and Screening
Nebulabio’s GFP-enabled screening platform accelerates early clone selection by enriching high-expression cell populations immediately after transfection or transduction. Single-cell FACS sorting ensures true monoclonality with complete electronic traceability, while non-invasive monitoring of GFP fluorescence during passaging enables real-time assessment of expression stability.
✎ Flp/FRT Recombinase-Based Rapid Gene Replacement
Nebulabio has developed platform host cell lines containing predefined FRT integration sites. Using Flp recombinase-mediated site-specific recombination, expression cassettes can be rapidly exchanged at an identical genomic locus. This approach minimizes clone-to-clone variability and ensures highly consistent expression across multiple projects, making it ideal for comparative studies and portfolio-scale development.
✎ Integrated End-to-End Service: From Concept to Clinic-Ready Cell Banks
Nebulabio provides a fully integrated solution covering the entire development lifecycle:
•Strategic consultation on host cell selection, expression system design, and vector optimization based on protein complexity and development stage
•Generation of two-tier master and working cell banks with comprehensive quality control, including identity testing, microbial safety testing, transgene verification, and long-term expression stability data
•Adaptation to serum-free suspension culture to enable seamless transfer to shake flasks and bioreactor processes
•Preparation of regulatory-ready CMC documentation supporting IND or CTA submissions, including development history, proof of monoclonality, and full characterization reports
▌ Proven Applications
Nebulabio’s stable cell line development platform has been successfully applied to the production of:
•Cytokines and growth factors such as Cardiotrophin-1 and Oncostatin-M
•Monoclonal antibodies and antibody fragments including IgG, scFv, and Fab
•Viral antigens and vaccine-related proteins
•Toxic or highly potent bioactive molecules
▌ Final Deliverables
Each project includes:
A fully characterized monoclonal research cell line
A comprehensive technical report detailing vector design, clone screening, growth performance, and stability studies
Cryopreserved vials of master and working cell banks with certificates of analysis
Detailed protocols for cell expansion, induction, and suspension adaptation
Partner with Nebulabio to Accelerate Your Biologics Pipeline
Nebulabio’s stable cell line development services are more than an outsourcing solution—they are a strategic partnership designed to reduce technical risk and accelerate development timelines. By combining engineering precision with regulatory awareness, we help our partners advance innovative biologics from concept to clinic with confidence.
Contact our cell line development experts today to discuss your target protein and learn how Nebulabio can unlock its full production potential.